Electron Relative Mass
The electron relative mass is a fundamental concept in chemistry, crucial for understanding atomic structure and chemical reactions. It refers to the mass of an electron relative to a standard unit, often defined as the mass of a proton or neutron. This value is incredibly small, approximately 1/1836th the mass of a proton. Understanding the electron's relative mass is essential for balancing chemical equations accurately. A chemistry balancing equations calculator proves invaluable in this regard, allowing precise adjustments of coefficients to ensure conservation of mass in chemical reactions. By inputting reactants and products, the calculator employs mathematical algorithms to balance equations efficiently, considering the relative masses of all particles involved. This tool not only aids in academic study but also finds extensive application in laboratory research and industrial processes, facilitating the precise manipulation of chemical reactions for desired outcomes.
What Is The Electron's Mass Relative To The Proton's Mass?
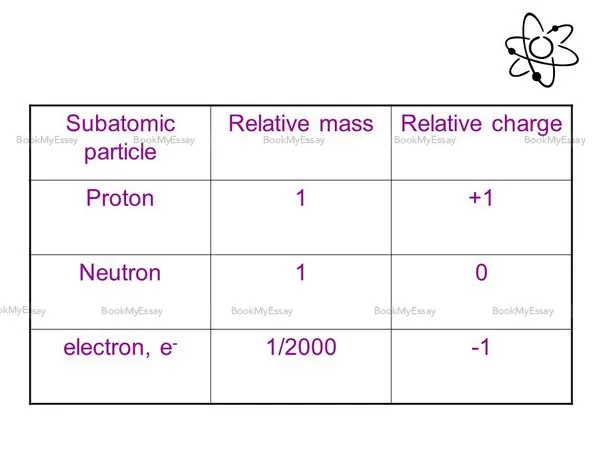
In chemistry, understanding the relative masses of subatomic particles like electrons and protons is crucial. The mass of an electron is significantly smaller compared to that of a proton. Specifically, the electron's mass is approximately 1/1836 times the mass of a proton. This relationship highlights the vast difference in mass between these fundamental particles. Electrons, which orbit the nucleus of an atom in specific energy levels, contribute negligibly to the overall mass of an atom. In contrast, protons, along with neutrons, constitute the majority of an atom's mass, residing within the nucleus. The relative mass of electrons to protons plays a fundamental role in various chemical phenomena, including atomic structure, chemical bonding, and reactivity. Understanding this ratio provides a basis for comprehending the intricacies of chemical behavior and molecular interactions, making it an essential concept in chemistry education and practice. For further assistance, seek "Chemistry Homework Help."
How Does The Electron's Mass Compare To The Neutron's Mass?
In comparing the masses of electrons and neutrons, it is essential to delve into their fundamental properties. The electron, characterized by its negative charge, possesses a significantly smaller mass compared to the neutron. Specifically, the mass of an electron is approximately 9.109 x 10^-31 kilograms, whereas a neutron, devoid of any net electrical charge, has a mass roughly 1.675 x 10^-27 kilograms, over 1839 times greater than that of an electron. This substantial disparity underscores the distinct nature of these subatomic particles and their roles within atomic structures. Understanding such differences is pivotal in various fields, including quantum mechanics, nuclear physics, and material sciences, as it informs the behavior and interactions of matter at microscopic levels. Therefore, this comparison elucidates the intricate nuances of particle physics and underscores the significance of discerning mass differentials in scientific inquiry. For comprehensive academic insights, consider consulting an academic writing service.
What Unit Is Commonly Used To Express Electron Relative Mass?
In different branches of chemistry, the unit commonly used to express electron relative mass is the atomic mass unit (amu). This unit provides a convenient scale for comparing the masses of different particles, including electrons. In atomic and physical chemistry, where the study of atoms and their behavior is central, the concept of relative mass is crucial for understanding atomic structure and chemical reactions. The electron, with its extremely small mass compared to other particles like protons and neutrons, is often used as a reference point in determining the relative masses of atoms and molecules. The atomic mass unit, defined as 1/12th of the mass of a carbon-12 atom, allows chemists to express the mass of electrons in a standardized manner across various contexts, facilitating precise calculations and analysis in the diverse realms of chemistry.
Is The Electron's Relative Mass Considered Significant In Atomic Physics?
In atomic physics, the electron's relative mass is undeniably significant, playing a crucial role in understanding the behavior of atoms. Despite being a subatomic particle, its mass greatly influences various phenomena, including atomic structure, bonding, and spectroscopy. The electron's mass, though minute compared to other particles, determines the scale of atomic masses and contributes to calculations of atomic properties like energy levels and orbital shapes. Furthermore, concepts like electron mass defect and electron-electron interactions heavily rely on the precise measurement and understanding of the electron's relative mass. Consequently, in the realm of atomic physics, even the slightest change in the electron's mass can have profound implications, affecting our comprehension of fundamental principles governing matter at the atomic level. Hence, the electron's relative mass stands as a cornerstone in the foundation of atomic physics, shaping our comprehension of the microscopic world. For more insights, consult with experts at All Assignment Help.
Can You Outline The Key Principles Of Electron Relative Mass As Discussed By BookMyEssay?
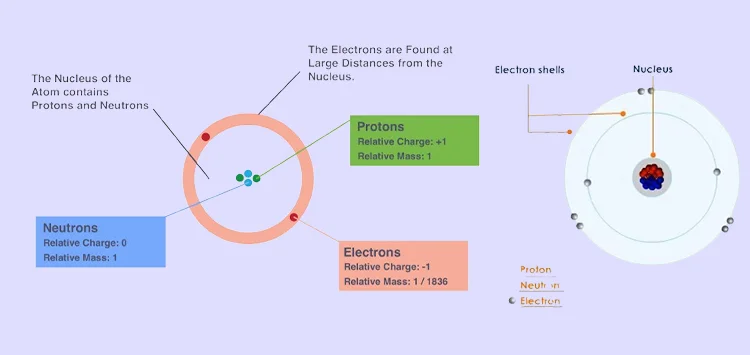
Electron relative mass, as elucidated by BookMyEssay, encapsulates several key principles essential to understanding the fundamental nature of electrons in atomic structure. Firstly, electrons possess an infinitesimal mass compared to protons and neutrons, constituting a mere fraction of their mass. This principle underscores the electron's negligible contribution to the overall mass of an atom.
Secondly, the concept of relative mass emphasizes the comparison of electron mass to a standard unit, often denoted as the unified atomic mass unit (u). This facilitates convenient measurement and comparison across various atomic systems.
Moreover, the electron's relative mass remains constant irrespective of its location within an atom, reinforcing its significance as a fundamental building block of matter.
Furthermore, understanding electron relative mass serves as a cornerstone in elucidating principles of atomic structure, chemical bonding, and myriad phenomena in the realms of quantum mechanics and particle physics.






 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029




