Crystal Structure Of A Diamond
The crystal structure of a diamond is a three-dimensional arrangement of carbon atoms bonded in a tetrahedral lattice. BookMyEssay elucidates this structure, emphasizing its unique properties and significance.
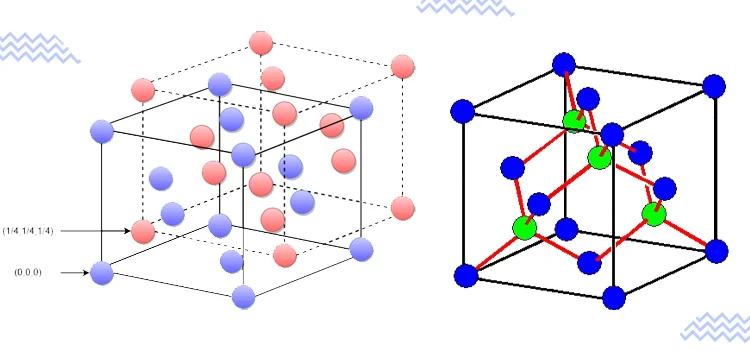
Diamonds have a cubic crystal system, with each carbon atom covalently bonded to four neighboring carbon atoms. This results in a strong and rigid structure, making diamonds one of the hardest natural substances.
BookMyEssay delves into the concept of diamond unit cells, illustrating how the arrangement of atoms repeats in three dimensions to form the crystal lattice. The diamond lattice is known for its symmetry, high density, and optical transparency.
Furthermore, our experts discuss the impact of impurities, defects, and crystal growth conditions on diamond structure and properties. Understanding the crystal structure of diamonds is crucial for various applications, including jewelry, cutting tools, electronics, and scientific research.
By providing insights into the crystal structure of diamonds, BookMyEssay enhances students' understanding of materials science, chemistry, and engineering concepts related to crystallography. We offer explanations, diagrams, and examples to facilitate learning and comprehension of this fascinating topic.






 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029




