What Are Protons Neutrons And Electrons
Protons, neutrons, and electrons are fundamental particles that constitute atoms, the building blocks of matter. Found within the nucleus of an atom, protons carry a positive electric charge, determining an element's identity. Neutrons, also in the nucleus, possess no charge, contributing to atomic mass without influencing the element's chemical properties. Electrons, meanwhile, orbit the nucleus in electron shells or energy levels, carrying a negative charge. These subatomic particles play crucial roles in defining the characteristics of elements and their behavior in chemical reactions.
Protons and neutrons combine to form the atomic nucleus, creating a positively charged core. Electrons, with their negative charge, are attracted to the positively charged nucleus, maintaining the stability of the atom. The number of protons in an atom defines its atomic number, distinguishing one element from another. Understanding the roles and interactions of protons, neutrons, and electrons provides the foundation for comprehending the intricacies of atomic structure and the diversity of elements in the periodic table.
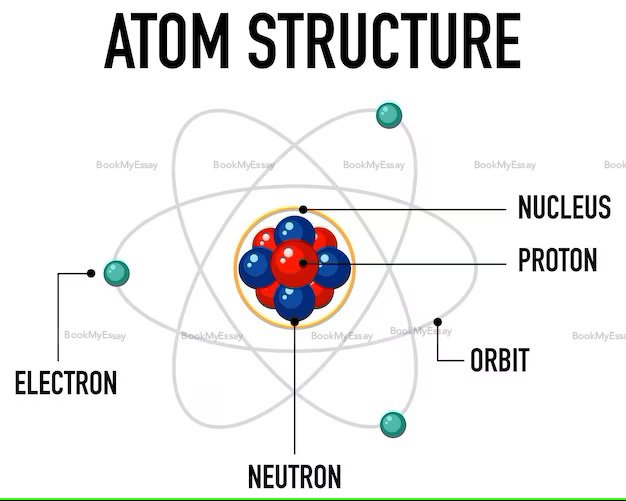
What Does "Atomic Structure" Actually Mean?
Understanding atomic structure is crucial in the realm of science and often becomes a focal point in students' science homework help. Atomic structure refers to the arrangement of particles within an atom, comprising protons, neutrons, and electrons. Protons and neutrons reside in the nucleus at the center, while electrons orbit in shells around it. This concept forms the basis for comprehending the properties and behavior of elements. When grappling with science homework, assignment help online can be invaluable in elucidating intricate details of atomic structure.
Online resources can aid students in grasping the fundamentals, such as the significance of the atomic number and mass number. The intricate dance of particles within an atom determines its chemical properties and reactivity. Delving into the depths of atomic structure not only enhances academic performance but also lays the foundation for a deeper understanding of the physical world. Seeking assignment help online can guide students through the nuances of atomic structure, transforming a challenging task into an enlightening learning experience.
When Was Atoms Discovered? The People Who Found The Electron, Proton, And Neutron?
The discovery of atoms marked a pivotal moment in the history of science assignment help, unraveling the fundamental building blocks of matter. In the late 19th and early 20th centuries, groundbreaking contributions from various scientists led to the identification of key atomic components. J.J. Thomson's experiments in cathode ray tubes unveiled the electron in 1897, demonstrating its role as a subatomic particle with a remarkably small mass compared to the atom. Ernest Rutherford, later, conducted the gold foil experiment, leading to the discovery of the proton in 1911. James Chadwick's work in the 1930s identified the neutron, completing the triumvirate of subatomic particles. Each particle had distinct properties, with the electron carrying a negative charge and having a relative mass much smaller than the proton and neutron. These discoveries laid the foundation for modern atomic theory, shaping our understanding of the microscopic world and influencing advancements in physics and chemistry.
What Are The Parts Of Atom?
An atom consists of three main components: protons, neutrons, and electrons. Protons carry a positive charge and are located in the nucleus at the atom's center, while neutrons, which are neutral, also reside in the nucleus. The nucleus, comprising protons and neutrons, forms the atom's core. Electrons, carrying a negative charge, orbit the nucleus in electron shells. These subatomic particles play crucial roles in determining an element's properties. Protons define an element's identity and contribute to its atomic number, while neutrons influence its stability. Electrons, positioned in specific energy levels, participate in chemical reactions and bonding.
When exploring the vast world of technology, particularly in web development, the mention of "Electron JS" often arises. This framework empowers developers to create cross-platform applications using web technologies like HTML, CSS, and JavaScript. Students grappling with Electron JS assignments can seek assistance through specialized services providing "Electron JS Assignment Help." These services aid in understanding the intricacies of Electron JS, ensuring a comprehensive grasp of this powerful tool for modern application development.
What Is The Relative Mass?
Relative mass refers to the comparison of the mass of an object to the mass of another object, usually taken as a standard reference. In the realm of academic assignments, understanding relative mass is crucial in physics and other scientific disciplines. Students often seek Assignment Help Online to grasp the concepts and applications of relative mass in various contexts.
In physics, relative mass is utilized to describe the mass of an object in relation to a standard mass, often denoted as the mass of an electron or proton. This comparison aids in understanding the scale and magnitude of different masses within a system. Academic assignment related to relative mass may involve calculations, analyses, and interpretations of experimental data. Online assistance for such assignments proves invaluable for students aiming to excel in their coursework, ensuring a comprehensive understanding of the principles governing relative mass and its significance in scientific research and problem-solving.
Struggling With Complex Chemistry Derivations? Call BookMyEssay’s Experts for Immediate Assistance
Struggling with complex chemistry derivations can be a daunting challenge, but fear not – BookMyEssay is here to provide immediate assistance from expert professionals. Our seasoned team is well-versed in the intricacies of chemistry, ready to guide you through the toughest derivations with ease. Whether you're grappling with organic, inorganic, or physical chemistry problems, our experts are equipped to offer comprehensive support.
Now, you might wonder, "What are the benefits of getting help with your science homework from Assignment Desk?" Well, the advantages are manifold. Firstly, our experts ensure a deep understanding of complex concepts, laying a solid foundation for future studies. Secondly, timely assistance guarantees on-time submission, reducing academic stress. Moreover, our tailored solutions cater to individual learning styles, enhancing comprehension and retention. So, if chemistry derivations are causing you distress, reach out to BookMyEssay's experts for personalized guidance and turn those academic challenges into opportunities for growth.







 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029




