How To Calculate Percent Error Chemistry
Calculating percent error in chemistry involves comparing experimental results with theoretical values. To ensure accuracy, utilize tools like a chemistry balancing equations calculator. Begin by subtracting the theoretical value from the experimental one, dividing the result by the theoretical value, and multiplying by 100. This yields the percent error, a crucial metric for assessing precision in scientific experiments. Students often encounter challenges in mastering this process, prompting the need for assignment writing help. Expert guidance can enhance comprehension of the intricacies involved, ensuring a thorough understanding of percent error calculations. By incorporating a chemistry balancing equations calculator and seeking assignment writing help, students can navigate the complexities of this fundamental aspect of chemical analysis, fostering a deeper grasp of scientific principles.
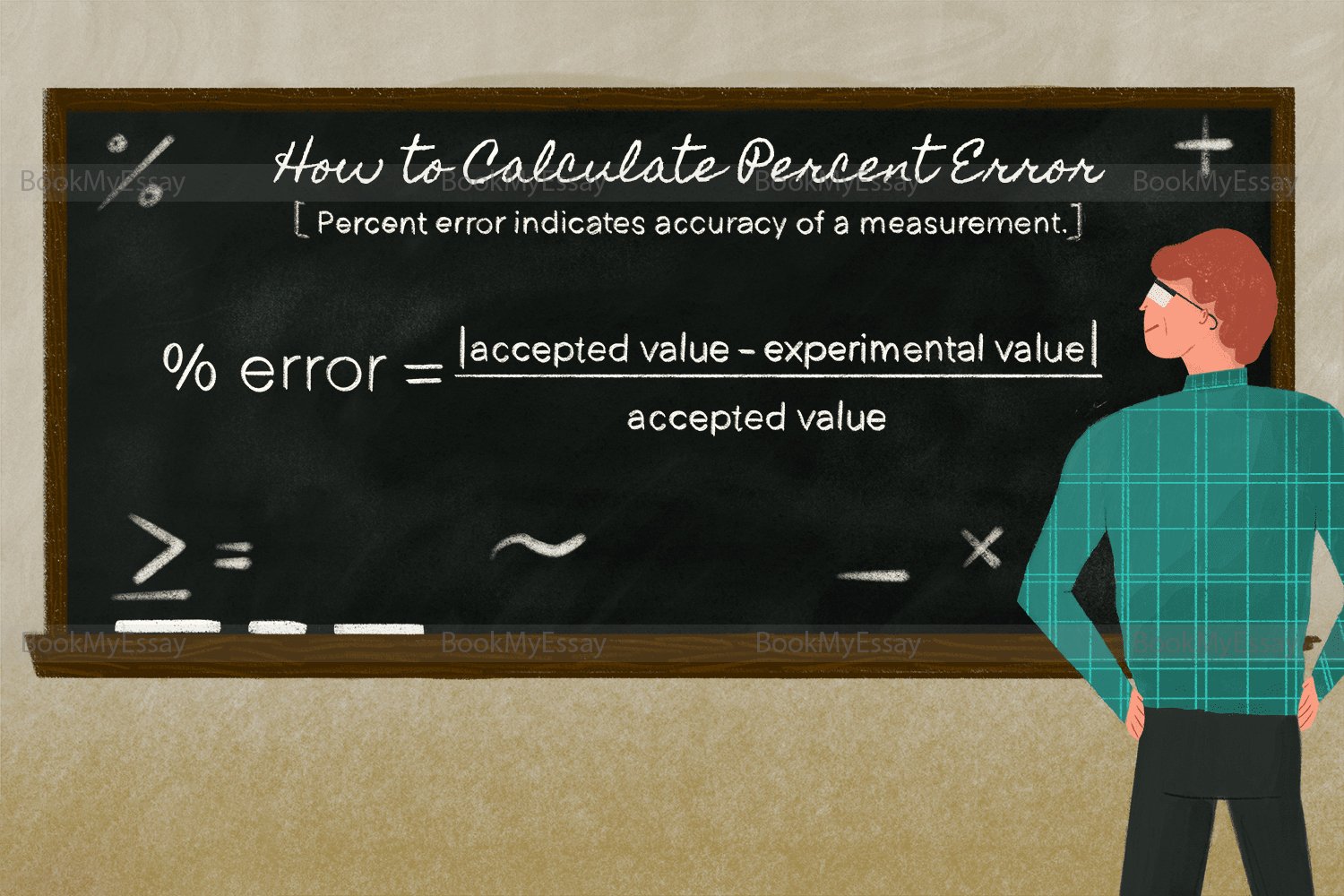
What Is The Formula For Calculating Percent Error In Chemistry?
In Chemistry coursework, calculating percent error is crucial for assessing the accuracy of experimental results. The formula for percent error involves comparing the experimental value to the accepted or theoretical value. Mathematically, it is expressed as [(Experimental Value - Theoretical Value) / Theoretical Value] * 100. This formula quantifies the discrepancy between the measured and expected outcomes, providing insights into the precision of laboratory techniques. Percent error serves as a valuable tool for students and researchers, guiding them in identifying and rectifying potential errors in their experimental procedures. A low percent error indicates a close alignment with theoretical predictions, reflecting a high level of accuracy. This fundamental concept underscores the importance of meticulous data analysis in Chemistry coursework, fostering a scientific approach to experimentation and analysis.
How Do You Determine The Experimental Value In Percent Error?
Determining the experimental value in percent error involves comparing measured results to accepted values, gauging the accuracy of an experiment. When seeking "cheap assignment help Australia," students may explore this concept. The formula for percent error is [(Experimental Value - Accepted Value) / Accepted Value] x 100. It quantifies the discrepancy between the obtained and expected results, aiding in the assessment of experimental precision. In the realm of affordable academic assistance in Australia, understanding percent error is crucial for scientific evaluations, fostering a culture of meticulous experimentation and analysis. Tutors providing cheap assignment help in Australia may emphasize the significance of accurate calculations, reinforcing foundational principles for students pursuing scientific disciplines, ensuring their academic success.
In Chemistry, What Is The Significance Of Calculating Percent Error?
In different branches of chemistry, calculating percent error holds paramount significance as it serves as a crucial metric to evaluate the accuracy of experimental results. Whether in analytical chemistry, physical chemistry, or organic chemistry, percent error provides a quantitative measure of the disparity between expected and observed values. This calculation aids researchers and scientists in assessing the reliability of their data, identifying sources of error, and refining experimental techniques. By determining the percentage difference, chemists can refine their methodologies, enhance precision, and ultimately contribute to the advancement of their respective fields. Percent error is a vital tool fostering precision and reliability across diverse branches of chemistry, ensuring that experimental outcomes align closely with theoretical predictions and fostering continual improvement in scientific methodologies.
What Role Does The Accepted Value Play In Percent Error Calculations In Chemistry?
In chemistry, the accepted value is a crucial component in percent error calculations. When students exclaim, "do my assignment," understanding this role becomes paramount. The accepted value represents the theoretically correct or standard measurement in an experiment. Percent error is calculated by comparing the experimental value (measured in the lab) to this accepted value. The formula involves subtracting the accepted value from the experimental value, dividing the result by the accepted value, and then multiplying by 100 to express the discrepancy as a percentage. This process assesses the precision and accuracy of experimental data. Students grappling with assignments should recognize that a lower percent error indicates greater accuracy, emphasizing the significance of the accepted value as a reference point for evaluating the reliability of experimental results in the realm of chemistry.
How Does BookMyEssay Help In Understanding Percent Error In Chemistry?
BookMyEssay serves as a valuable resource for students grappling with the intricacies of percent error in chemistry. The platform offers expertly crafted study materials and guidance tailored to demystify this challenging concept. Through detailed examples and clear explanations, BookMyEssay elucidates the nuances of calculating and interpreting percent error in chemical analyses. Its user-friendly interface allows students to access comprehensive notes, practice problems, and step-by-step solutions, fostering a deeper understanding of the subject. Additionally, the platform provides personalized assistance, ensuring that students can clarify doubts and reinforce their knowledge. By bridging the gap between theory and application, BookMyEssay plays a pivotal role in enhancing students' comprehension of percent error in chemistry, ultimately contributing to their academic success and confidence in the field.







 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029




