Formation Of Ions By Electron Loss or Gain Assignment Help
For students grappling with the intricacies of "Formation of Ions by Electron Loss or Gain," seeking specialized "Assignment Help" is a wise choice. This topic delves into the fundamental concept of ions emerging through electron manipulation, a cornerstone of chemistry. It explores how atoms shed or acquire electrons, leading to charged particles with distinct chemical behavior.
However, comprehending this phenomenon can be challenging, prompting the need for reliable "Academic Writing Help." Professional assistance offers a structured approach, simplifying complex theories and mechanisms. Expert guidance aids in dissecting the processes of electron loss (cation formation) and gain (anion formation), elucidating factors like electronegativity and ionization energy.
A proficient "Assignment Help" service ensures comprehensive coverage of this subject, addressing students' queries while enhancing their analytical and writing skills. By receiving tailored support, learners can grasp the nuances of ion formation, paving the way for a strong foundation in chemistry. So, for those navigating the labyrinth of "Formation of Ions by Electron Loss or Gain," availing oneself of credible "Academic Writing Help" can be the key to conquering the academic challenges effectively.
What is The Formation Of Ions By Electron Loss or Gain?
Are you seeking assistance with understanding the concept of ion formation through electron loss or gain? Our online platform offers comprehensive Assignment Help Online services, including specialized guidance like Electron JS Assignment Help.
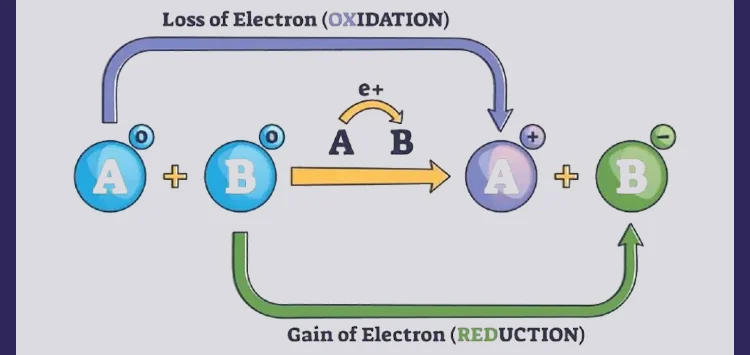
When atoms undergo a process of ionization, they can either gain or lose electrons. This results in the formation of ions, which are charged particles due to an imbalance between protons and electrons. When an atom loses one or more electrons, it becomes a positively charged ion, known as a cation. Conversely, if an atom gains electrons, it becomes a negatively charged ion, called an anion.
Electron loss occurs when atoms have a lower ionization energy, making it easier for them to shed electrons and achieve a more stable configuration. On the other hand, atoms with a higher electron affinity tend to gain electrons and achieve a full outer electron shell, enhancing their stability.
Understanding the nuances of ion formation, electron loss, and gain can be challenging, but our Assignment Help Online platform, including specialized Electron JS Assignment Help, is here to provide you with the guidance and support you need to grasp these fundamental concepts effectively.
Objectives Of Formation Of Ions By Electron Loss or Gain
The formation of ions through electron loss or gain serves crucial objectives in the realm of chemistry. Assignment Writing Help Tutors elucidate these concepts for students seeking clarity. When atoms either lose electrons to become positively charged cations or gain electrons to transform into negatively charged anions, several pivotal goals are achieved.
Firstly, this phenomenon leads to the attainment of stable electronic configurations akin to the noble gases, enhancing an atom's overall stability. Secondly, it facilitates the establishment of ionic compounds through electrostatic attractions between positively and negatively charged ions, resulting in the formation of diverse and robust chemical bonds. This understanding, often offered by Assignment Writing Help Tutors, is vital for comprehending the properties and behavior of various substances.
Furthermore, the manipulation of ions by either electron loss or gain allows for the development of innovative materials and technologies, underpinning advancements in fields like medicine, electronics, and energy storage. Students seeking a comprehensive grasp of these principles can Buy Assignment Help Online, accessing valuable resources and personalized guidance. In essence, the objectives of ion formation through electron loss or gain extend beyond theoretical knowledge, influencing practical applications that shape modern scientific and technological landscapes.
Why Students Can Rely On BookMyEssay For Assignment Help
As an esteemed assignment help provider, BookMyEssay has emerged as a dependable resource for students seeking assignment help in the UK. With a reputation for excellence, it offers a wide array of benefits that make it a reliable choice.
Firstly, BookMyEssay boasts a team of skilled and experienced writers who specialize in various subjects. This ensures that students receive well-researched and meticulously crafted assignments tailored to their academic needs.
Secondly, the platform places a premium on timely submissions. Students can trust BookMyEssay to deliver assignments promptly, saving them from last-minute stress and allowing them to focus on other aspects of their studies.
Furthermore, the service ensures originality by providing plagiarism-free content. This is crucial in maintaining academic integrity and earning top grades.
Moreover, the user-friendly interface of the platform makes it easy for students to place their assignment orders and communicate directly with writers.
Lastly, the customer support team is available 24/7 to address any queries or concerns, enhancing the overall experience for students.
BookMyEssay stands as a reliable assignment help provider in the UK, offering expertise, punctuality, originality, user-friendliness, and exceptional customer support.






 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029




