Examples Law Of Conservation Of Mass
The Law of Conservation of Mass is a fundamental principle in chemistry, stating that the total mass of substances involved in a chemical reaction remains constant before and after the reaction, provided no mass is lost or gained from the system. This law is exemplified in various scientific phenomena, such as combustion reactions, where the mass of the reactants equals the mass of the products. For instance, when wood burns, the mass of the ash, smoke, and gases formed equals the initial mass of the wood and oxygen consumed.
Linked to this principle is the Law of Conservation of Energy, which states that energy cannot be created or destroyed, only transformed from one form to another. While the Law of Conservation of Mass deals with matter, the Law of Conservation of Energy pertains to energy. Both laws are interconnected and crucial in understanding physical and chemical changes.
For assistance in comprehending these scientific principles or completing assignments related to them, seeking help with assignments online from reliable educational platforms or tutors specializing in these topics can provide valuable guidance and support.
What Role Does The Conservation Of Mass Law Play In Physical Systems?
The Conservation of Mass Law stands as a fundamental principle in physical systems, asserting that within a closed system, mass remains constant over time despite any transformations or reactions it undergoes. This law aligns with the Conservation of Energy definition, which states that energy cannot be created or destroyed within an isolated system; rather, it transforms from one form to another.
In various physical processes like chemical reactions, nuclear reactions, or even natural phenomena such as combustion or photosynthesis, the Conservation of Mass Law plays a pivotal role. It serves as a critical tool for understanding and predicting the outcomes of these processes, enabling scientists and engineers to calculate the quantities of reactants and products accurately.
For students seeking Homework Writing Help, comprehending this law aids in solving problems related to mass changes during reactions or transformations. Understanding this principle allows for the formulation of equations and predictions, facilitating a deeper grasp of the physical sciences and their applications in real-world scenarios. The Conservation of Mass Law remains a cornerstone in explaining the stability and predictability of physical systems across various disciplines.
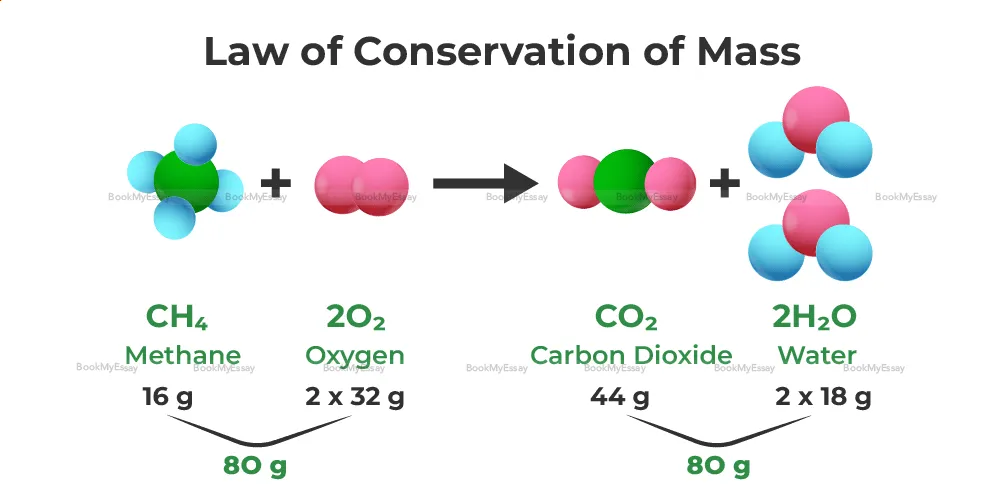
Could You Briefly Describe The Law Of Conservation Of Mass Concept?
The Law of Conservation of Mass is a fundamental principle in chemistry stating that the mass of substances remains constant before and after a chemical reaction, provided there is no loss or gain of matter from the system. In simpler terms, it asserts that matter cannot be created or destroyed, only transformed from one form to another.
Exploring this concept can lead to interesting science research paper topics. Some potential areas include investigating historical developments in understanding conservation laws, exploring the practical applications of the law in industries such as pharmaceuticals or environmental science, or delving into the connection between the conservation of mass and energy in the context of Einstein's theory of relativity.
For students seeking Assignment Help Online, understanding this principle lays a strong foundation for comprehending various chemical reactions and their quantitative aspects. Additionally, discussing real-world applications or conducting experiments to illustrate this law could make for engaging assignments, fostering a deeper understanding of this fundamental concept in science.
How Does The Conservation Of Mass Law Affect Various Types Of Energy?
The conservation of mass law fundamentally influences various forms of energy across scientific disciplines. This principle, rooted in physics and chemistry, stipulates that mass cannot be created or destroyed but can only transform from one form to another during physical or chemical changes. This law interconnects with diverse energy types, impacting their conversion and utilization.
In the realm of academic writing help and custom assignment writing services, this law's implications are significant. It underpins discussions on energy transformation in papers tackling topics like thermodynamics, chemical reactions, and environmental studies. For instance, in thermodynamics essays, the conservation of mass law underscores how energy changes from potential to kinetic or thermal forms, emphasizing its preservation within a closed system.
Moreover, in chemistry assignments, it's pivotal to elucidate how different energy types align with this law during chemical reactions and conversions of matter. Understanding this principle is indispensable for accurate analysis and interpretation, forming a cornerstone for comprehensive academic discussions on energy within various disciplines. Hence, the conservation of mass law stands as a fundamental pillar, shaping the discourse surrounding energy dynamics in academic contexts, shaping the discourse in custom assignment writing services and academic writing help.
Which Mass Conservation Principles Are Addressed In The BookMyEssay Assignment?
BookMyEssay, a renowned assignment help provider, intricately addresses several principles of mass conservation in its online assignment writing help service for university students. The assignments offered by BookMyEssay meticulously delve into the core principles of mass conservation, encompassing fundamental concepts like the law of conservation of mass and energy.
The experts at BookMyEssay elucidate the significance of conserving mass in various disciplines, be it physics, chemistry, or environmental sciences. They elucidate the law of conservation of mass that states that in a closed system, mass remains constant over time, implying that mass cannot be created or destroyed, only transformed or transferred.
Moreover, the assignments provided by this service outline practical applications of mass conservation in real-life scenarios. From chemical reactions to ecological systems, the comprehensive assistance offered by BookMyEssay empowers students to comprehend and analyze mass conservation principles, ensuring a profound understanding and application of these concepts in academic pursuits and practical domains.







 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029



