Are you looking for a Lewis Dot structure but still not finding guidance on how to draw Lewis Dot structure? If yes, so you reached in right place BookMyEssay is here for your n2 Lewis Dot Structure assignment help. We’ll provide you with a complete service of the Lewis Dot structure method. Here you can get the Do My Assignment for me service.
Basically, the Lewis dot structure refers that the electron outermost shell which is also known as valence electrons, and potential covalent bonds in an atom or molecule. All electron valence are poorly charged and attracted the charged positive nucleus which is made up of neutrons and protons. Always remember that the electron is moving constantly around the nucleus and it is not contained in one place as shown in the 2D structure.
It is a series of dots, lines, and atomic symbols and gives a structure to the way an atom or molecule is arranged in this series. The creation of a Lewis dot structure for a single atom and covalent compound. For more details read this blog till the end.
Let’s check through the Periodic Table how you can make a Lewis Dot Structure
The Scientific Periodical table also helps you to create a Lewis Dot structure. Every group or column in a periodic table is indicated by a Roman number that represents the series of valence electrons. This table will apply to the whole group of the table. For example, all the main elements of the atom that fall in the first column or group I have one (1) valence electron. All elements in group II have two (2) valence electrons and up to eight (8) valence electrons. Atom properties are also consistent across rows, or periods, of the periodic table. Periods are represented by a number, 1, 2, 3, so on. these represent the level of energy or electron shells. The first period, or row, has only one energy level that can hold a total of two electrons. Period 2, with a second shell, can hold a total of eight (8) electrons, also known as the octet rule.
The periodic table also informs you about the process of electronegativity. In the table, the elements of electronegativity are situated in the top-right corner of the scientific periodical table and it will decrease the power of electronegativity elements when you move down in the group or move towards the left of a period.
While drawing or making Lewis dot structures, the scientific periodical table will be very helpful for your reference point when you are working with electrons, covalent bonds, and so on.
Have some important steps of how you can easily Build a Lewis Dot Structure
Step 1. First, you have to determine the total series of valence electrons that can be easily represented in the diagram of Lewis.
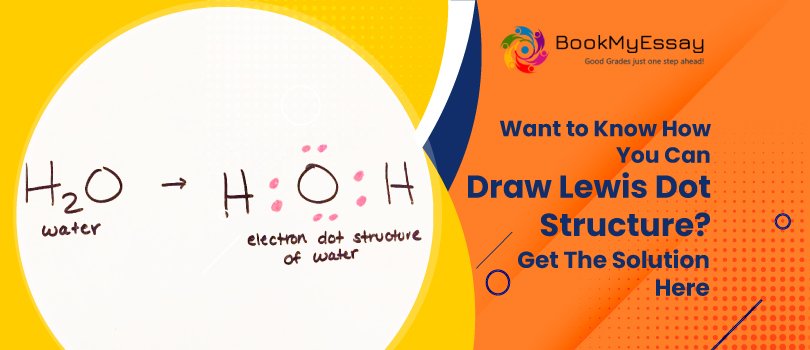
For example: H2O total = 18
Step 2. After that, you can see the last element of electronegative in the middle and make single bonds from the middle atom to another atom.
Step 3. Then you have to determine the total number of electrons that should be added to the middle element.
Step 4. You have to make double or triple bonds to the atom of central until it makes an octet
Step 5. In the end, you will add all the outer elements of Electrons till all the elements have to Completed the Octals process.




 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029




