Sulfur tetrafluoride's formula is SF4. This chemical is a gas that has no color. Furthermore, it is regarded as a top-tier organic fluorinating agent. Many people wonder if SF4 is polar or nonpolar. In this blog, we'll look at the answer to this question, as well as its properties and how it can be used. BookMyEssay is the�homework help service.
Difference Between Polar and Non-Polar Molecules
There are different forms of forces that bind the molecules. These forces include ionic bonds, covalent bonds, metallic bonds, and hydrogen bonding. The molecules that are covalently bonded can be polar and nonpolar. Let us discuss what differences occur between polar and non-polar molecules. If you need SF4 Polar or Non-Polar Assignment Help, then BookMyEssay is the cheapest solution.
Polar Molecules:
- Molecules containing these atoms do not have a uniform distribution of charge. These molecules always have a dipole moment greater than 0.
- As a result of the unequal electronegativity of atoms in these molecules, each atom influences the bonded electron pair differently.
- Covalent bonds formed between two atoms are generally polar if their electronegativity values differ.
- In general, polar molecules have asymmetric geometry.
- Hydrogen, sulfide, and oxygen are some examples of these molecules.
Nonpolar Molecules:
- Molecules with a uniform distribution of charges over their atoms are called non-polar molecules.
- Since both atoms have an equal share of bonded electron pairs, the electronegativity of nonpolar covalent bonds is equal.
- In general, symmetry is observed in the geometry of nonpolar molecules. Some examples of such molecules are XeF2, O2, and so on.
Identifying the Polarity of a Compound
Electronegativity: An atom's ability to draw a bonded pair of electrons is referred to as its electronegativity. Higher an atom's electronegativity, the more electrons it attracts.
If there is a difference between the electronegativity of two atoms that make up a molecule, the resulting molecule will be polar because its atoms will have unequal amounts of charge. Polarity is directly proportional to electronegativity difference and vice versa. BookMyEssay provides�expert assignment writing help to the students.
However, in the case of symmetric geometry, molecules with unequal electronegativity can still be nonpolar. This is because the dipoles in such molecules are canceled out by each other as a result of the symmetric shape.
Geometric shape: It is also used to determine if a compound is polar. Due to the uneven distribution of charges, polar molecules typically have an asymmetrical shape. In contrast, nonpolar molecules have symmetrical shapes.
A molecule can be nonpolar even if its constituent atoms have different electronegativity if its bonds are symmetric and its dipoles are canceled out by those of neighboring bonds.
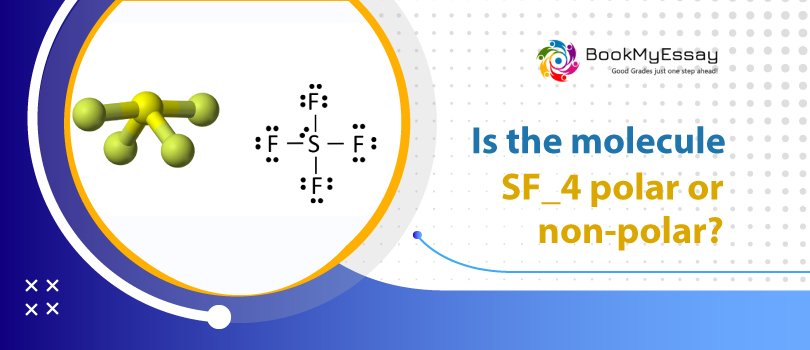
Is SF4 a Polar or Non-Polar Compound?SF4 contains a lone pair on its sulfur atom, making it polar and giving the molecule an asymmetric, seesaw-like shape. In addition, fluorine has a higher electronegative potential than sulfur does. Because of this, the overall charge distribution within a molecule is not evenly distributed.�As we've seen, the shape of a molecule can tell us a lot about its polarity.
The sulfur atom has 1 lone pair, so SF4 is asymmetric, like a seesaw. Fluorine is more electronegative than sulfur, so it attracts the bonded electron pair slightly more strongly toward itself and acquires a partial negative charge, while sulfur acquires a partial positive charge.
Charge distribution on Sulfur tetrafluoride atoms becomes irregular. When fluorine is in the middle, it acts as a negative pole, and Sulfur tetrafluoride turns into a positive pole.�The dipole moment value of the Sulfur tetrafluoride is 0.632 D.
Qualities of BookMyEssay
BookMyEssay is a trusted assignment help service that annually assists thousands of chemistry students from all academic levels and specializations. When they return to finish another assignment during the following semester, those students do so again. BookMyEssay gives you reliable and high-quality online engineering assignment help that is easy to get.





 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029



