A Covalent Bond Is Likely To Be Polar When
"A Covalent Bond Is Likely To Be Polar When" delves into the fascinating realm of chemical bonding, shedding light on the conditions under which covalent bonds exhibit polarity. Authored by experts in the field and available through Assignment Help Tutors, this insightful resource navigates through the nuances of molecular interactions.
Within its pages, readers uncover the fundamental principles governing covalent bonds and the factors influencing their polarity. From electronegativity differences between atoms to molecular geometry, each concept is elucidated with clarity and precision. Through illustrative examples and in-depth explanations, this book equips students and enthusiasts alike with a comprehensive understanding of chemical bonding.
Moreover, "A Covalent Bond Is Likely To Be Polar When" serves as a valuable companion for those seeking to deepen their knowledge in chemistry. Whether tackling academic assignments or exploring the intricacies of molecular structures, this resource proves indispensable.
Accessible through Assignment Help Tutors, students can easily access this resource to enhance their learning experience. With its user-friendly format and rich content, this book is an essential addition to any chemistry enthusiast's library. Dive into its pages and unravel the mysteries of covalent bonding like never before.
What Elements Influence A Covalent Bond's Polarity?
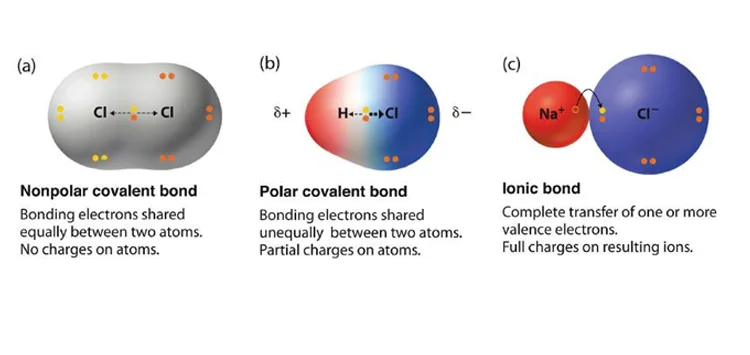
Understanding the factors that influence a covalent bond's polarity is crucial in chemistry. Several elements play significant roles in determining whether a covalent bond is polar or nonpolar. One such element is electronegativity. Electronegativity refers to an atom's ability to attract electrons towards itself in a chemical bond. When two atoms with different electronegativities form a covalent bond, the shared electrons are not equally distributed, leading to a polar bond. Additionally, molecular geometry influences bond polarity. Even if the atoms involved have similar electronegativities, the bond may still be polar if the molecule's shape causes an uneven distribution of charge.
Furthermore, the presence of lone pairs of electrons on atoms within the molecule can also affect bond polarity. Lone pairs can create regions of higher electron density, leading to polar bonds. Other factors such as bond length and molecular symmetry can also contribute to bond polarity.
For students seeking comprehensive insights into these concepts, resources provided by Coursework Writers can be invaluable. Understanding these elements empowers students to predict molecular behavior accurately and comprehend the properties of various compounds. Mastery of these fundamental principles not only enhances academic performance but also lays a solid foundation for further studies and applications in chemistry.
What Effect Does The Difference In Electronegativity Have On Bond Polarity?
The disparity in electronegativity between atoms in a chemical bond profoundly influences its polarity, a crucial concept explored in Homework Writing Help and Assignment Help resources. Electronegativity, a measure of an atom's tendency to attract shared electrons, dictates the distribution of electron density within a molecule.
When there's a substantial difference in electronegativity between bonded atoms, one atom exerts a stronger pull on the shared electrons, leading to an uneven distribution of charge. Consequently, the atom with higher electronegativity acquires a partial negative charge (δ−), while the other atom develops a partial positive charge (δ+). This results in the formation of a polar covalent bond, characterized by unequal sharing of electrons.
Conversely, when the electronegativity difference is minimal or nonexistent, electrons are shared equally between atoms, resulting in a nonpolar covalent bond.
Understanding the effect of electronegativity on bond polarity is pivotal in predicting the behavior of molecules in various chemical reactions and environments. It influences properties such as solubility, melting and boiling points, and intermolecular forces.
In Homework Writing Help and Assignment Help materials, this concept is elaborated upon with illustrative examples and practical applications, empowering students to grasp the intricacies of molecular interactions and their implications in chemistry.
How Does Bond Polarity Relate To The Idea Of Molecular Geometry?
Bond polarity and molecular geometry are closely interconnected concepts in chemistry, both playing crucial roles in determining the overall behavior and properties of molecules. When considering the relationship between bond polarity and molecular geometry, it's essential to understand that molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. This arrangement is influenced by the number of bonding and non-bonding electron pairs around the central atom.
The distribution of electrons within a molecule, affected by bond polarity, influences its shape. For instance, polar covalent bonds result from the unequal sharing of electrons between atoms with different electronegativities. This uneven distribution of charge creates partial positive and negative poles within the molecule. Molecular geometry, on the other hand, determines how these polarities are arranged in space.
Assignment Help in UK facilitates understanding this intricate relationship by providing expert guidance and resources to students tackling complex topics like bond polarity and molecular geometry. Through comprehensive assistance, students can grasp the theoretical foundations and practical applications of these concepts, aiding them in their academic endeavors and future careers in chemistry.
BookMyEssay provides exploring the connection between bond polarity and molecular geometry, students gain insight into how the structural arrangement of atoms influences a molecule's polarity and overall behavior, laying the groundwork for a deeper understanding of chemical interactions and compounds.
Popular Subject
- Cheap Homework Help
- Do My Essay Online
- London Assignment Writing Help
- Write My Assignment for Me
- A+ Certified Professional Assign...
- Yahoo! Query Language Assignment...
- Competition and Consumer Law Ass...
- Law Dissertation Assignment Help
- History Homework Writing Help
- Bamboo Flooring Assignment Help
- AP Style Writing Assignment Help
- Business Cycle Assignment Help
Rating
4.9/5
5 Star Rating

Charles
Australia
Rating:
Everything is good and helpdesk supports is cooperative, all problems of my assignment are solved perfectly.

Johnson
USA
Rating:
Thank you BookMyEssay for all your great services. I am so happy that I get this assistance with my study.




 3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029
3 Bellbridge Dr, Hoppers Crossing, Melbourne VIC 3029




